WO 2015097605
Mylan Laboratories Ltd.
Process for the preparation of sofosbuvir
02 July 2015
The present disclosure relates to processes for the preparation of sofosbuvir or of its pharmaceutically acceptable salts. The present disclosure also provides intermediates useful in the synthesis of sofosbuvir.
Kaushik, Vipin Kumar; Vakiti, Srinivas; Ravi, Vijaya Krishna; Tirumalaraju, Bhavanisankar
Nucleoside phosphoramidates are inhibitors of RNA-dependent RNA viral replication and are useful as inhibitors of HCV NS5B polymerase, as inhibitors of HCV replication and for treatment of hepatitis C infection in mammals.
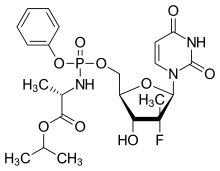
Sofosbuvir (PSI-7977) is a nucleotide analog inhibitor of HCV NS5B polymerase, which is developed by Pharmasset and used for the treatment of chronic hepatitis C (CHC) infection as a component of a combination antiviral treatment regimen. SOVALDI® tablets contain sofosbuvir, which is chemically named as (S)-Isopropyl 2-((S)-(((2R,3R,4R,5R)-5-(2,4-dioxo3,4-dihydropyrimidin-l(2H)-yl)-4-fluoro-3-hydroxy-4-methyltetrahydrofuran-2yl)methoxy)-(phenoxy)phosphorylamino) propanoate and is represented by the following chemical structure:

Formula-1 Sofosbuvir and a process for the preparation are disclosed in U.S. Patent No. 7,964,580 B2 and PCT Publication No. WO 2008/121634 A2, which are hereby incorporated by reference. The present disclosure provides a novel process for the preparation of sofosbuvir or its pharmaceutically acceptable salts that employs novel intermediates.
SUMMARY OF THE DISCLOSURE
A first aspect of the present disclosure is to provide a process for the preparation of sofosbuvir or its pharmaceutically acceptable salts. In one embodiment, the present disclosure provides a process for the preparation of sofosbuvir or its pharmaceutically acceptable salts that includes the steps of: a) reacting the compound of formula 4 with a compound of formula 5 to get a compound of formula 3;

4 b) hydrolyzing the compound of formula 3 to get a compound of formula 2; and

3 2 c) optionally deprotecting the compound of formula 2 to get sofosbuvir of formula 1 or its pharmaceutically acceptable salts.

1 2 wherein R is hydrogen or any hydroxy protecting group and X is a leaving group such as tosylate, camphorsulfonate, mesylate, trifluoroacetate, trifluorosulfonate, an aryloxide, heteroaryl oxide or an aryloxide or heteroaryl oxide substituted with at least one electron-withdrawing group. In another embodiment, the present disclosure provides a novel intermediate of formula 3a.

In an additional embodiment, the present disclosure provides a crystalline compound of formula 3a, which is characterized by a powdered X-ray diffraction pattern as shown in Figure 1. In September 2014, Gilead entered into non-exclusive licensing agreements with various generic companies (including Mylan) to manufacture and supply generic sofosbuvir. In April 2015, Mylan launched its generic version of the drug as MyHep, in India
scheme-II.

Sofosbuvir Scheme-II In another embodiment the present disclosure provides a process for the preparation of sofosbuvir as shown in below
scheme-Ill.

Example 3: Preparation of sofosbuvir (formula 1). N-Benzoyl Sofosbuvir (6 g) was added to 70% w/w aqueous acetic acid (90 mL) and the contents were stirred at 90-95 °C. After completion of the reaction, which was monitored by qualitative HPLC, the reaction mass was cooled to ambient temperature, diluted with water and filtered through a Hyflo filter.
Thereafter, obtained filtrate was extracted with ethyl acetate which was further washed with ~4%w/w aqueous hydrochloric acid followed by ~9%w/w aqueous sodium carbonate solution. Finally, the ethyl acetate layer was washed with water and dried.
The dried layer was concentrated under reduced pressure at 60-65 °C. Thereafter, the concentrated mass was dissolved in a mixture of 5% isopropanol in methylene dichloride and isopropyl ether was added to precipitate the product. After stirring at 0-5 °C for 2 hours, the product was filtered, washed with methylene dichloride/isopropyl ether mixture, which was recrystallized with methylene dichloride/isopropyl ether mixture to yield sofosbuvir as white crystals (3 g)......https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2015097605&recNum=1&maxRec=&office=&prevFilter=&sortOption=&queryString=&tab=PCTDescription

Mylan launches Sovaldi tablets in India
Sovaldi is indicated for the treatment of chronic hepatitis-C infection as a component of a combination antiviral treatment
Pharma giant Mylan NV today said its subsidiary Mylan Pharmaceuticals has launched Gilead Sciences' Sovaldi (sofosbuvir 400mg tablets) in the country.
Sovaldi is indicated for the treatment of chronic hepatitis-C infection as a component of a combination antiviral treatment.
It is estimated that around 12 million people are chronically infected with hepatitis-C in India, Mylan said in a release.
In February this year, Gilead appointed Mylan as its exclusive distributor of Sovaldi in India.
Mylan president Rajiv Malik said they have a history of partnering with Gilead to tackle key public health issues in India and around the world, beginning with expanding access to high quality and affordable HIV/AIDS antiretrovirals.
"We are proud to continue our work together with the launch of Sovaldi as it supports our joint commitment to meeting the unmet medical needs of patients in India," Malik said.
Gregg Alton, Executive Vice-President, Corporate and Medical Affairs, Gilead Sciences said it makes an important milestone in the company's ongoing effort to make its hepatitis-C medicines accessible to as many patients, in as many places, as quickly as possible.Sovaldi is sold by Mylan's dedicated sales force as part of its Hepato Care segment.

,,,,,,,,,,
 amcrasto@gmail.com
amcrasto@gmail.com
 LIONEL MY SON
LIONEL MY SON
He was only in first standard in school when I was hit by a deadly one in a million spine stroke called acute transverse mylitis, it made me 90% paralysed and bound to a wheel chair, Now I keep him as my source of inspiration and helping millions, thanks to millions of my readers who keep me going and help me to keep my son happy































 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..