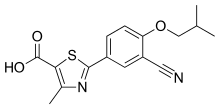
The present invention relates to an improved process for the preparation of anti- hyperuricemia drug 2-[3-cyano-4-(2-methylpropoxy)phenyl]-4-methylthiazole-5- carboxylic acid (commonly known as Febuxostat) and its pharmaceutically acceptable salts, represented by the following structural formula- 1.
Formula- 1
Further, the present invention provides novel amine salts of compound of formula- 1 and its process for the preparation.
The present invention also provides a novel process for the preparation of crystalline forms A, B and G of Febuxostat. Febuxostat is an inhibitor of xanthine oxidase that is indicated for use in the treatment of hyperuricemia and gout.
Febuxostat was approved by the European Medicines and the U.S. Food and Drug Administration. Febuxostat is marketed by Takeda Pharmaceuticals with the brand names Adenuric (EU) and Uloric (US).
Background of Invention:
2-[3-cyano-4-(2-methylpropoxy)phenyl]-4-methylthiazole-5-carboxylic acid was first disclosed in US 5614520. The disclosed process comprises of reacting the ethyl 2-[4- hydroxy-3-nitrophenyl]-4-methyl-5-thiazolecarboxylate with 1 -bromo-2-methylpropane in the presence of potassium carbonate in dimethyl formamide provides ethyl 2-[4-(2- methylpropoxy)-3-nitrophenyl]-4-methyl-5-thiazole carboxylate which is reduced with Pd/C and the obtained compound is treated with sodium nitrite in water and followed by a mixture of cuprous cyanide and potassium cyanide provides ethyl 2-[4-(2- methylpropoxy)-3-cyanophenyl]-4-methyl-5-thiazolecarboxylate. The obtained ethyl 2- [3-cyano-4-(2-methylpropoxy)phenyl)-4-methyl-5-thiazolecarboxylate was hydrolyzed by treating with aqueous sodium hydroxide in a mixture of ethanol and tetrahydrofuran provides 2-[3-cyano-4-(2-methylpropoxy)phenyl]-4-methylthiazole-5-carboxylic acid having melting range of 238 - 239°C with the yield of 33%. The above process utilizes Pd/C, which is difficult to handle in the laboratory, hence it is not recommended on large scale. However, the yield and purity were also not satisfactory. The said process involves more number of steps, hence there is a need to produce the process for the preparation of 2-[3-cyano-4-(2-methylpropoxy)phenyl]-4-methylthiazole-5-carboxylic acid with less number of steps.
Heterocycles, 1998, Vol. 47, No. 2, 857-864 disclosed a process for the preparation of 2-[3-cyano-4-(2-methylpropoxy) phenyl]-4-methylthiazole-5-carboxylic acid which comprises of reacting 4-(2-methylpropoxy)-l,3-benzenedicarbonitrile in the presence of hydrochloric acid with thioacetamide in dimethyl formamide provides 4-(2- methylpropoxy)-3-cyanobenzthioarnide. It was reacted with ethyl 2-chloroacetoacetate in ethanol provides ethyl 2-[4-(2-methylpropoxy)-3-nitrophenyl]-4-methyl-5-thiazole carboxylate which is further hydrolyzed with aqueous sodium hydroxide solution in a mixture of tetrahydrofuran and ethanol provides 2-[3-cyano-4-(2-methylpropoxy) phenyl]-4-methylthiazole-5-carboxylic acid with the yield of 35% which on further recrystallized from acetone provides a colorless crystals having the melting range of 201 - 202°C. However the yield and purity were not satisfactory. JP10-045733 disclosed a process for the preparation of 2-[3-cyano-4-(2- methylpropoxy) phenyl]-4-methylthiazole-5-carboxylic acid which comprises of reacting ethyl 2-(3-formyl-4-hydroxyphenyl)-4-methylthiazole-5-carboxylate with hydroxyl amine in 2011/000328 presence of formic acid and sodium formate provide ethyl 2-(3-cyano-4-hydroxyphenyl)-4- methylthiazole-5-carboxylate. The obtained compound was reacted with l-bromo-2- methylpropane in presence of potassium carbonate provides ethyl 2-[3-cyano-4-(2- methylpropoxy) phenyl]-4-methylthiazole-5-carboxylate, which on further hydrolysis with aq sodium hydroxide provides 2-[3-cyano-4-(2-methylpropoxy) phenyl]-4-methylthiazole-5- carboxylic acid.
2-[3-cyano-4-(2-methylpropoxy) phenyl]-4-methylthiazole-5-carboxylic acid produced by the above processes contains 2-[3-carbamoyl-4-(2-methylpropoxy)phenyl]-4- methylthiazole-5-carboxylic acid as an impurity. So there is a need for the purification to get the highly pure 2-[3-cyano-4-(2-methylpropoxy) phenyI]-4-methylthiazole-5-carboxylic acid.
WO2011141933
In general, febuxostat prepared by the reported processes contain impurities like amide impurity, ethyl ester impurity and methyl ester impurity etc. It is important that any pharmaceutical compound or intermediate should free of impurities or if present they must be limited to the maximum level set by ICH guidelines. The purification technique of by recrystallisation of the compound from a suitable solvent such as the recrystallisation of febuxostat as disclosed in Heterocycles, Vol. 47, No. 2, 1998 does not yields the desired purity. Hence it is necessary to have an alternate purification technique for the preparation of highly pure febuxostat.
In the aforementioned processes using aqueous sodium hydroxide for hydrolyzation of ethyl 2-[4-(2-methylpropoxy)-3-nitrophenyl]-4-methyl-5-thiazole carboxylate, which lead to the formation of impurities such as ethyl ester impurity, methyl ester impurity, amide impurity and high polar impurities. Hence there is a need to develop a process for the preparation of highly pure febuxostat with high yield.
The crystalline forms A, B, C, D and G of febuxostat and their preparation were first disclosed in US 6225474. However as on date, there is no alternative processes were reported in the prior art for preparing the said polymorphs. Henceforth, there is a need to develop a novel process for the preparation of said crystalline polymorphs.
In view of the foregoing, there is a necessity for the improved process which overcome the problems of prior art and to produce the highly pure 2-[3-cyano-4-(2- methylpropoxy) phenyl]-4-methylthiazole-5-carboxylic acid with high yield.
Advantages of Present Invention:
• Avoids the usage of Pd/ C .
• Provides the highly pure 2-[3-cyano-4-(2-methylpropoxy)phenyl]-4-methyl-5- thiazole carboxylic acid with high yield.
• Controls the impurities (such as methyl impurity, ethyl impurity, amide impurity and high polar impurities) levels to meet the ICH guidelines.
• Provides a novel process for the preparation of crystalline forms A, B and G of 2-[3- cyano-4-(2 -methylpropoxy) phenyl]-4-methyl-5-thiazolecarboxylic acid.
· Provides a novel and improved processes for the preparation of 4-(2-methylpropoxy)- 1,3 -benzene dicarbonitrile and also provides a simplest process for the purification of 2-[3-cyano-4-(2-methylpropoxy)phenyl]-4-methyl-5-thiazole carboxylic acid.
• Eco-friendly process.
• Uses simple, milder reagents which are easier to handle and use in large scale.
WO2011141933
Formula-6 ForrauIa-
Scheme-II:
Scheme-Ill:
Example 8: Preparation of Febuxostat (Formula-1)
A mixture of compound of formula-5 (50 g), sodium hydroxide (23.25 g), tetrahydrofuran (250 ml) and water (13 ml) was heated to 60-65°C and stirred for 8 hours. After completion of the reaction, the reaction mixture was cooled to 25-30°C and quenched it with water. The reaction mixture was stirred for 1 hour at 25-30°C. A solution of hydrose (2.5 g) in water (25 ml) was added to the reaction mixture at 25-30°C and stirred for 30 minutes. The pH of the reaction mixture was adjusted to 1.3 with hydrochloric acid. The reaction mixture was extracted thrice with ethylacetate, washed with water and the organic layer was dried with sodium sulfate. Carbon (2.5 g) was added to the organic layer and stirred for 30 minutes. The reaction mixture was filtered through hyflow bed and washed with ethylacetate. Distilled off the solvent completely from the obtained filtrate to get the solid. Ethyl acetate (200 ml) was added to the obtained solid and heated to reflux temperature and stirred for 30 minutes. The reaction mixture was cooled to 25-30°C, filtered the solid obtained and washed with ethyl acetate. Acetone (250 ml) was added to the wet solid obtained, heated to 55-60°C and stirred for 15 minutes. Water (250 ml) was added to the reaction mixture and stirred for 2 hours. The obtained solid was filtered, washed with aqueous acetone and then dried to get the title compound. Yield: 38 grams.
Example 9: Preparation of Febuxostat (Formula-1)
A mixture of compound of formula-5 (50 g), sodium hydroxide (23.25 g), tetrahydrofuran (250 ml) and water (13 ml) was heated to 60-65°C and stirred for 8 hours. After completion of the reaction, the reaction mixture was cooled to 25-30°C and quenched it with water. The reaction mixture was stirred for 1 hour at 25-30°C. A solution of hydrose (2.5 g) in water (25 ml) was added to the reaction mixture at 25-30°C and stirred for 30 minutes. The pH of the reaction mixture was adjusted to 1.3 with hydrochloric acid. The compound was extracted thrice with ethyl acetate, washed with water and the organic layer was dried with sodium sulfate. Carbon (2.5 g) was added to the reaction mixture and stirred for 30 minutes. The reaction mixture was filtered through hyflow bed and washed with ethylacetate. Distilled off the solvent completely from the obtained filtrate to get the solid. Acetone (250 ml) was added to the solid obtained, heated to 55-60°C and stirred for 15 minutes. Water (250 ml) was added to the reaction mixture and stirred for 45 minutes . Cooled the reaction mixture to 25-30°C and stirred for 30 minutes, further to 0-5 °C and stirred for 2 hours. The obtained solid was filtered, washed with aqueous acetone and then dried. Ethylacetate (200 ml) was added to the obtained solid and heated to reflux temperature and stirred for 30 minutes. The reaction mixture was cooled to 25-30°C. Filtered the solid, washed with ethyl acetate and then dried to get the pure title compound. Yield: 39 grams
Example 10: Preparation of ethyl 2-[3-formyl-4-(2-methyIpropoxy) phenyl]-4- methylthiazole-5-carboxylate compound of formula-12.
To a stirred solution of ethyl 2-[3-formyl-4-hydroxyphenyl]-4-methylthiazole-5- carboxylate (10 gms) in dimethylformamide (40 ml) added potassium carbonate (9.78 gms) and potassium iodide (2.35 gms). Heated the reaction mixture to 70 - 75°C and stirred for 30 minutes. To the above reaction added a solution of l-bromo-2- methylpropane (9.72 gms) in dimethyl formamide (10 ml) and stirred for 5 hours. Cooled the reaction mixture to 25°C, quenched with water and stirred for one hour. Filtered the precipitated solid and dried the material to get the title compound. Yield: 9 gms.
Example 11: Preparation of ethyl 2-[3-cyano-4-(2-methyIpropoxy) phenyl]-4- methyIthiazoIe-5-carboxylate compound of formuIa-5.
To the solution of ethyl 2-[3-formyl-4-(2-methylpropoxy) phenyl]-4- methylthiazole-5-carboxylate compound of formula-12 (10 gms) in formic acid (40 ml) was added hydroxylamine hydrochloride (2.38 gms) and sodium formate (2.35 gms).
Stirred the reaction mixture for 10 minutes. Heated the reaction mixture to 100°C and stirred for four hours at same temperature. Cooled the reaction mixture to 25 °C and quenched with water. Stirred the reaction mixture for 10 hours, filtered the precipitated solid and washed with water. Dried the material to get the title compound. Yield: 10 gms. Take the dry material, added 30 ml of ethyl acetate and heated to reflux temperature. Stirred the reaction mixture for 30 minutes at reflux temperature. Cooled the reaction mixture to 25°C and filtered the precipitated solid. Dry the material to get the title compound as a pure material. Yield: 8.5 gms.
| CN101497589A * | Feb 26, 2009 | Aug 5, 2009 | 沈阳药科大学 | Method for synthesizing 2-(3-cyano-4- isobutoxy phenyl)-4-methyl-carboxylate |
| CN101863854A * | Jun 29, 2010 | Oct 20, 2010 | 沈阳药科大学 | Synthesis method of 2-(3-cyan-4-isobutoxy) phenyl-4-methyl-5-thiazole formic acid |
| JP2706037B2 * | | | | Title not available |
| Citing Patent | Filing date | Publication date | Applicant | Title |
|---|
| WO2012131590A1 * | Mar 28, 2012 | Oct 4, 2012 | Sandoz Ag | An improved process for preparation of febuxostat and its polymorphic crystalline form c thereof |
| WO2014009817A1 * | Mar 19, 2013 | Jan 16, 2014 | Alembic Pharmaceuticals Limited | Pharmaceutical composition of febuxostat |
| WO2014057461A1 | Oct 10, 2013 | Apr 17, 2014 | Ranbaxy Laboratories Limited | Process for the preparation of crystalline form g of febuxostat |

MORE................
Febuxostat is an inhibitor of
xanthine oxidase, and was developed by
Teijin pharma. This compound is known as a new drug that is effective against gout and
hyperuricemia, and it has been 40 years since the last time a drug of this kind of drug was developed.
Febuxostat has therefore gained a lot of popularity and it has already been accepted as a drug in Europe, USA, Korea and Japan. The synthesis of this molecule have been reported in patents by Teijin pharma as shown below.[1,2]
Recently, Itami group was reported the rapoid synthesis of febxostat by using Ni-catalyzed direct coupling of azoles and arylhalides[3]
Sorbera, L.A.; Castaner, J.; Rabasseda, X.; Revel, L.; TMX-67. Drugs Fut 2001, 26, 1, 32
[1] Hasegawa, M.; A facile one-pot synthesis of 4-alkoxy-1,3-benzenedicarbonitrile. Heterocycles 1998, 47, 2, 857. [2] Hasegawa, M.; Hasegawa, M.; Komoriya, K. (Teijin Ltd.); Cyano cpds. and their preparation method. JP 1994345724 . [3] “Nickel-Catalyzed Biaryl Coupling of Heteroarenes and Aryl Halides/Triflates”
Canivet, J.; Yamaguchi, J.; Ban, I.; Itami, K.
Org. Lett. 2009,
11, 1733-1736. DOI:
10.1021/ol9001587
Ni-based catalytic systems for the arylation of heteroarenes with aryl halides and triflates have been established. Ni(OAc)2/bipy is a general catalyst for aryl bromides/iodides, and Ni(OAc)2/dppf is effective for aryl chlorides/triflates. Thiazole, benzothiazole, oxazole, benzoxazole, and benzimidazole are applicable as heteroarene coupling partners. A rapid synthesis of febuxostat, a drug for gout and hyperuricemia, is also demonstrated.
SEE,,,,,,,,http://www.allfordrugs.com/2016/07/10/febuxostat/
/////////