
SILDENAFIL
http://www.google.co.ug/patents/US20010009962
This invention relates to a process for the preparation of 1-[[3-(6,7-dihydro-1-methyl-7-oxo-3-propyl-1H-pyrazolo[4,3-d]pyrimidin-5-yl)-4-ethoxyphenyl]sulfonyl]-4-methylpiperazine(otherwise known as sildenafil or Viagra™), and 1-Ethyl-4-{3-[3-ethyl-6,7-dihydro-7-oxo-2-(2-pyridylmethyl)-2H-pyrazolo[4,3-d]pyrimidin-5-yl]-4-propoxyphenylsulphonyl}piperazine and key intermediates thereof.
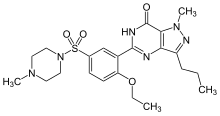
[0002] 1-[[3-(6,7-dihydro-1-methyl-7-oxo-3-propyl-1H-pyrazolo[4,3-d]pyrimidin-5-yl)-4-ethoxyphenyl]sulfonyl]-4-methylpiperazine (otherwise known as sildenafil) has been found to be particularly useful in the treatment of, inter alia, male erectile dysfunction (WO-A-94/28907), and a process for its preparation is disclosed in EP-A-0463756 (example 12) and Drugs of the Future 1997, 22(2): 138-143. An improved process for preparing sildenafil (over that of EP0463756) is disclosed in EP-A-0812845, with the characterising final step involving cyclisation under basic, neutral or acidic conditions to form sildenafil. A process for the preparation of 1-Ethyl-4-{3-[3-ethyl-6,7-dihydro-7-oxo-2-(2-pyridylmethyl)-2H-pyrazolo[4,3-d]pyrimidin-5-yl]-4-propoxyphenylsulphonyl}piperazine is disclosed in WO 98/49166 (example 5B).
[0003] The present inventors have now found a process for preparing sildenafil and 1-Ethyl-4-{3-[3-ethyl-6,7-dihydro-7-oxo-2-(2-pyridylmethyl)-2H-pyrazolo[4,3-d]pyrimidin-5-yl]-4-propoxyphenylsulphonyl}piperazine which has advantages over the aforementioned prior art processes.
[0004] According to the present invention there is provided a process for preparing a compound of formula (IA) and (IB)
[0005] comprising reacting a compound of (IIA) and (IIB) respectively in the presence of −OR, wherein R in the case of formation of compound (IA) is CH2CH3 and R in the case of formation of compound (IB) is CH2CH2CH3, where X is a leaving group:
[0006] A particular advantage of the present process over that of the prior art is the elimination of steps by carrying out a substitution reaction and ring closure in ‘one pot’.
[0007] The intermediates of general formula (IIA) and (IIB) form a further aspect of the invention.
[0008] A key intermediate of the general formula (IIIA) and (IIIB) (see schemes 1 and 2 hereafter) have been identified in various reactions showing that such reactions at least partially go via a pathway of cyclisation then nucleophilic substitution. Accordingly intermediates of general formula (IIIA) and (IIIB) form yet a further aspect of the invention (wherein X is a leaving group).
[0009] A further major intermediate of formula IVA and IVB have also been identified, suggesting that there is also nucleophilic substitution before cyclisation (and these intermediates, where novel, form a further aspect of the invention).
[0010] Thus the proposed reaction pathways for the formation of compounds (IA) and (IB) are as follows
[0061] (1e) 1-[[3-(6,7-dihydro-1-methyl-7-oxo-3-pronyl-1H-pyrazolo[4,3-d]pyrimidin-5-yl)-4-ethoxyphenyl]sulfonyl]-4-methylpiperazine. (Compound IA)
[0062] Potassium t-butoxide (0.74 g, 6.60 mmol) was added to a suspension of the title compound of example (1d) (1.00 g, 2.20 mmol) in ethanol (5 ml) and the mixture was heated under reflux for 48 hours. The reaction mixture was stripped down to an oil and purified by dissolving in dichloromethane and washing with saturated sodium bicarbonate solution. Hexane was added to the organic solution over 10 minutes, a precipitated solid filtered and dried to afford the title compound (1.1 g, 100%) as a white solid. Recrystallisation of the title compound from ethyl acetate affords a solid with m.p. 184-186° C. Found: C, 55.49; H, 6.35; N, 17.72. C22H31N6O4S requires C, 55.58; H, 6.53; N, 17.68. δ (DMSO): 0.96 (3H, t), 1.30 (3H, t), 1.72 (2H, m), 2.13 (3H, s), 2.36 (4H, m), 2.72 (2H, t), 2.90 (4H, m), 4.18 (5H, m), 7.32 (1H, d), 7.80 (2H, m). m/z (Found: 475.214800 ([M+H]+, 100%). C22H31N6O4S. requires 475.212751).




