- What is intellectual property?
Intellectual property (IP) can be defined as any novel or previously undescribed invention, process, machine, composition of matter, life form, article of manufacture, software, data, written work, design or image, and know-how and information associated with the above. Intellectual Property may or may not be protectable under legislation, and may be tangible (e.g. biological organisms, plant varieties, computer software, engineering drawings etc.) or intangible (e.g. patents, copyrights, ideas, tacit knowledge etc.). IP is owned by individuals, organizations, institutions etc., and can be bought, sold or licensed. It is therefore essential that IP be adequately protected.
Patents, registered designs, trade marks and copyright are the most common ways of protecting IP and preventing others from using or otherwise exploiting it without the owner's permission. Other types of IP include circuit layout rights, plant breeders' rights and trade secrets. Although each is a separate area of law, all are designed to provide some protection against the unauthorized use of products of the human mind, particularly if such use provides an unfair trade advantage.
IP allows people to own their creativity and innovation in the same way that they can own physical property. The owner of IP can control, and be rewarded for, its use, and this encourages further innovation and creativity to the benefit of us all. - What constitutes an invention?
An invention is a novel and useful idea relating to processes, machines, manufactures, and compositions of matter. It is probable that an invention has been made when something new and useful has been conceived or developed, or when unusual, unexpected, or non-obvious results have been obtained and can be exploited. An invention may be the product of a single individual or a group of individuals who have collaborated on a project. - Who is an inventor?
According to US patent law, inventors are those who have made independent, original, conceptual contributions to an invention. For the purposes of its Intellectual Property Policy, the MRC refers to individuals that contribute to inventions as either IP creators or IP enablers. IP Creator(s) are individuals who are deemed to have made an intellectual contribution to the creation and/or development of IP. They do not include individuals that have only carried out the tasks or supplied materials, and are not necessarily those appearing as authors on a scientific publication. IP Enabler(s) are individuals who have indirectly contributed to the creation and/or application of Intellectual Property and without whose intellectual contribution commercial application would not have been possible. Assistants, technicians and others who have contributed in taking the idea or concept to fruition may be considered as IP Enablers. IP enablers do not necessarily qualify as inventors for patenting purposes. - Who owns the invention?
As described in the MRC's Intellectual Property Policy, the MRC, in most cases, owns all IP generated by MRC-supported researchers. Where IP emanates from external MRC units, the IP is jointly owned with the relevant university. The IC acts on behalf of the MRC and the researcher(s) to transfer inventions to the marketplace. Any revenue generated from the successful transfer of the invention is then divided proportionately between the IC, the MRC, the MRC unit where the invention was made, and the researcher(s).
All about Patents and Intellectual property by DR ANTHONY MELVIN CRASTO, Worldpeaceambassdor, worlddrugtracker, Ph.D ( ICT, Mumbai) , INDIA 30+Yrs Exp. in the feld of Organic Chemistry, Serving chemists around the world. THE VIEWS EXPRESSED ARE MY PERSONAL AND IN NO-WAY SUGGEST THE VIEWS OF THE PROFESSIONAL BODY OR THE COMPANY THAT I REPRESENT, amcrasto@gmail.com, +91 9323115463 India
HOW TO FILE INDIAN PATENT
- Home
- ABOUT ME
- GOOGLE SITE
- PATENT SEARCH ENGINE
- Patent Download Websites
- SIPO CHINA PATENT SEARCH
- WIPO
- Glossary
- IP AUSTRALIA
- FREE ONLINE PATENTS
- Intellectual Property in China
- PATENT OFFICES
- Indian patent search
- Patent expiry search
- Korea patent search in English
- HOW TO LOOK FOR PATENT TERM EXTENSION, PTA
- HOW TO FILE INDIAN PATENT
- HOW TO PREPARE PATENT LANDSCAPE
- HOW TO SEARCH PATENT FAMILY
Saturday, 13 December 2014
INTELLECTUAL PROPERTY, INVENTIONS AND INVENTORS
TECHNOLOGY TRANSFER - THE BASICS

THE BASICS
- What is technology transfer?
Technology transfer is the process of transferring laboratory research outputs from the bench to the marketplace, usually through partnerships with industry. - What is the role of the MRC IC in technology transfer and how can we help MRC scientists?
The MRC IC is committed to developing a culture of innovation within the MRC's science base. The Centre's primary function is to manage the development and transfer of sustainable health care technologies to industry for the benefit of the MRC, MRC researchers, and society in general. The IC also ensures that the inventors and their respective units receive a portion of any benefits arising from the commercialization of their inventions. (Click here for more information on the MRC IC and its functions) - What is the role of MRC scientists/inventors in technology transfer?
The assistance and commitment of the inventor are vital to the technology transfer process. With the passing of the IPR Act in 2008, and in line with the MRC's Intellectual Property Policy, all MRC-supported researchers are obliged to disclose any inventions to the IC and to cooperate with them as far as possible in all matters pertaining to technology transfer. The expertise and knowledge of the inventor are essential to the IC for assessing the potential applications, market and patentability of the invention and to commercial partners for product development and manufacture. MRC researchers are also responsible for keeping thorough records of their experiments in order to assist in the technology transfer process and in the legal protection of the invention (See Guidelines for Laboratory Notebooks for more information). - How can MRC scientists benefit from technology transfer?
As documented in the MRC's Intellectual Property Policy, IP creators/enablers will receive a portion of the net income generated from the successful transfer of their inventions to the marketplace. A further portion of the income is awarded to the Unit in which the IP creators/enablers work.
PROTECTING INTELLECTUAL PROPERTY

PATENTS
- What is a patent?
A patent is an exclusive right granted for a specific period of time to an inventor, or other entitled person(s), in exchange for a full disclosure of the invention to the public. A patent is property and may be sold by "assignment" (i.e. transferring all rights to another party) or licensed for use by others via a license agreement in which the patentee retains all rights of ownership, but the right to use the invention is licensed out for a fee.
Patents provide the patent owner with the right to exclude others from exploiting the invention for the life of the patent. Generally patents are granted for 20 years, starting from the date on which the first patent application is filed. Patent rights are territorial, which means that a South African patent, for example, does not afford rights outside of South Africa.
It is important to note that what is granted is not the right to make, use, offer for sale, sell or import, but the right to exclude others from making, using, offering for sale, selling or importing the invention. Once issued, the patentee must enforce the patent without the aid of the Patent Office. - What are the legal requirements for patenting an invention?
In order for an invention to be patentable, it must be novel, have utility, and differ from what skilled users in the art or technology might expect (non-obvious). An idea cannot be patented if it was known to the public, published, or secretly applied before the application date. The invention must have an inventive step, must have an application within industry, agriculture or commerce, and must add value and benefit society in some way. - What constitutes public disclosure?
Any disclosure of an invention to the public prior to the filing of a patent will essentially result in the invention no longer being patentable. Public disclosure includes dissemination of information on the invention (a sufficiently detailed description of the invention that allows it to be duplicated or put into use) through newspaper articles, newsletters, bulletins, textbooks, journals, theses, reports, letters to journal editors, oral presentations etc.
Specific types of disclosure to guard against are:- informal discussions outside of your unit/institute
- postings on the web
- talks at meetings
- chats with non-MRC colleagues
- abstracts
- posters
- unprotected e-mail
- In what categories are patents granted?
- Processes/methods - a form of treatment of certain materials to produce a specific result
- Machines - a mechanical device or combination of mechanical devices to perform some function and produce a certain result
- Articles of manufacture - any tangible item not already included in the process or machine description (this is essentially a catchall category)
- Components of matter - covers all products whether the result of a chemical mixture or mechanical mixture or other compounds
- Improvements of the previous categories - an improvement may be an addition to, simplification or alteration of any of the above
- What is patentable in the field of biotechnology?
The following are patentable:- Non-living entities - DNA, recombinant DNA genes, promoters, plasmids, vectors, polypeptides, antibodies that are present in organisms
- Living entities - Genetically modified organisms and plant and animal cultures.Researchers should make sure that they are in possession of the isolated/purified entity prior to seeking patent protection.
- What is not patentable?
The following are not patentable:- Discoveries, i.e. natural phenomena that had to have existed previously in order to be discovered
- Scientific theories, i.e. theories that define what already exists
- Mathematical methods
- Presentations of information
- Methods of performing a mental act, or of doing business
- Methods for treatment of a human or animal body by means of surgery or therapy
- Plants and animals which have not been genetically modified
- Biological processes
- The metabolism of organisms
- Can genes, ESTs or SNPs be patented?
The patentability of various forms of genetic information remains uncertain and may depend on the usefulness of the information. Patents on genes that code for medically useful proteins such as erythropoeitin and thrombopoietin have been granted. The situation is less clear for open reading frames of unknown function or for expressed sequence tags (ESTs) and single nucleotide polymorphisms (SNPs). If it can be shown that genes, ESTs or SNPs are associated with a particular disease in a way that enables diagnosis of that disease or susceptibility to disease, then this may enable patenting. Similarly, if a gene encodes a protein that possesses a beneficial function or is a drug target for the treatment of a disease, this may also allow patenting.
Presently, different patent offices around the world are governed by their own patentability criteria when it comes to genetic information. The European Patent Office (EPO) and the US Patent Office, for example, differ with regard to their stance on the patentability of ESTs and SNPs. Currently, the US Patent Office will grant a patent on an EST sequence, as long as evidence for a credible utility (such as its use as a probe for a particular disease gene) can be provided. - Can computer-related (bioinformatics) inventions be patented?
Computer programs are patentable so long as they generate the ability for the computer to perform a novel technical function. For example, a program that simply sorted something into alphabetical order, which can be done manually, is unlikely to be patentable. Applications of computation are also patentable. For example, a patent has been granted for a computerised method and system for the analysis of an electrophoresis gel test. - Who should be listed as an inventor on a patent?
According to US patent law, inventors are those who have made independent, original, conceptual contributions to an invention. More specifically, it defines inventors as those who have made at least one contribution to the patent claims (i.e. the legal definitions of an invention) included in the patent application. When two or more persons contribute jointly to a patentable discovery, all inventors must participate in the patent application or risk the patent's invalidation. A university, corporation, or other business association may not apply in its own name as an inventor but is registered with the Patent Office as the owner (assignee or applicant) of the patent.
Inventorship should be determined upfront to avoid any conflict with regard to personal recognition, royalty payments, license fees etc. The acknowledgement of IP creators and IP enablers and their relative contributions to the invention are provided for on the MRC's Invention Disclosure Form and Intellectual Property Contributors Form. - How does the patenting process work?
10.1. Filing a provisional application
The so-called "patent prosecution" process usually begins with preparing and filing a provisional patent application with a local or international Patent Office. A provisional application is usually filed when the invention is still under development and is likely to be changed or improved over the next year. Patent application forms differ for each patent office but most contain the following headings:
Individual inventors are allowed to file their own patent applications, however, due to the specific and often complex rules associated with patents and their contents and the fact that patents are legal documents, it is advisable to enlist the assistance and advice of a patent attorney. The patent attorney and the inventor(s) work together to draught the patent application, which is similar to a detailed scientific paper and must contain a full description of the invention that allows others to make and use it as well as a clear definition of the boundaries of the invention.
- Field of the invention
- Background of the invention
- Objectives of the invention
- Summary of the invention
- Detailed description of the invention and description of the drawings
- Claims (not included in provisional applications)
10.2. The priority date
The date on which the patent application is filed is referred to as the priority date, i.e. the date from which the invention is deemed to have existed and from which the invention is protected. The priority date is important for determining priority in cases where similar inventions are patented. The applicant is given world-wide pending rights for one year from the priority date.
The 12 months following the priority date can be used for further development of the idea, to assess the commercial potential of the invention, and to identify how the invention will be exploited. It is possible to file additional data relating directly to the patent application as separate applications during this time. If after 12 months it is clear that the invention cannot be exploited, the patent can be abandoned or withdrawn.
At the end of the twelve-month period, a complete international patent specification has to be filed. This will include any further applications filed or data collected during the priority year. The contents of the application cannot be amended beyond this date, however, if the idea is developed further, the original patent can be abandoned and a new one filed. The most common route after the provisional patent is to file a PCT application.
10.3. The Patent Co-operation Treaty
The international Patent Co-operation Treaty (PCT) allows you to file one international patent application that offers provisional protection in a number of different countries (PCT Contracting States) designated by you. Patents must be filed directly with countries that are not included in the PCT. Filing a PCT application buys you 18 months of extra time to evaluate the marketability of the invention before incurring huge patenting costs involved in the "national phase".
10.4. International Search Report
Usually 4-5 months after filing a PCT application you will receive an international search report listing any prior art documents relevant to your claims that have been identified by the International Searching Authority. This will give you some idea of the novelty and inventiveness (how non-obvious it is) of your invention. If the prior art questions the novelty and/or inventiveness of your invention, you can amend the claims to better distinguish your invention from prior art or withdraw the application before it is published.
You also have the option of obtaining an international preliminary examination report, which will give you some information on the patentability of your invention before going through the expensive patent granting procedure in each of the countries you selected. PCT applications are examined by individuals with expertise in the invention's technical field to determine whether the invention is patentable and whether it is novel, and an Examination Report is issued after 28 months (from the priority date). Certain claims in the patent application may be found to be patentable, whilst others might be rejected. The applicant is able to file responses and amendments to counter the arguments for the rejection of claims.
10.5. Other important patenting timelines
Approximately 18 months after the first filing date the patent is published, together with the international search report. If you decide to withdraw a patent, it should be done before it is published in order to maintain secrecy.
If you have filed a provisional patent application, you can claim the priority date of the provisional patent provided that the PCT is filed within 12 months of the priority date. Otherwise the priority date will be the date of filing of the international application.
10.6. The national phase
A PCT application secures you the right to seek patent protection in a number of countries; however, the actual granting of a patent remains the responsibility of the national patent offices, and happens during the "national phase." After a period of 30 months from filing the provisional patent application, or 18 months after filing a PCT application, the applicant must move into the "national phase," i.e. file complete patents in each of the countries in which protection is required. This can be extremely costly since national fees must be paid in each country and the patents must be translated into the language of each country involved and must be examined for compliance with the local patent law.
The inventor should therefore be certain of the potential financial returns from the invention before entering this phase.
Each national patent office will conduct a full examination of the patent to determine novelty, utility and inventiveness.
The international search and preliminary international examination during the PCT phase will facilitate the prosecution process in the national phase, since you would already have made the necessary adjustments to address the prior art identified and the patentability issues. The applicant is again able to make amendments to the patent to address any objections raised in the examination report. The patent will either be approved for grant once all the objections have been addressed or it will be abandoned. Please note that even if a patent has been granted, a third party may still challenge its validity. Once a patent is granted, it will be subject to annual renewal fees in each of the countries in which it is filed.
Under the European Patent Convention patent protection can be obtained in 19 countries by filing a single application at the European Patent Office. Six other Extension states are expected to become members in due course. Similarly, by filing a single application with ARIPO or OAPI one can obtain protection in a number of African countries.
10.7. Patent prosecution and protection period
It typically takes 3-10 years from the initial filing for a patent to be granted, depending on the territory and ease of examination. The invention will be protected for 20 years from the filing date. The patenting process is represented schematically in the figure above.
10.8. Patenting costs
The average costs for filing and maintaining a patent are provided in the table below. These costs do not include fees for possible official action (reporting and responding), grant and issue of patents, examination requests, validation costs, translation costs etc.
Estimated patenting costs for South African inventors
Application Average Filing Cost Average Annual Renewal Fees SA Provisional R7,000 – R15,000 - PCT R45,000 – R90,000 - SA Complete R10,000 – R20,000 R800 National USA R60,000 – R80,000 R5,000 National Europe R60,000 – R100,000 R5,000 – R10,000/country
- Who benefits from a patent?
The granting of a patent and the successful transfer of that invention benefits everyone concerned. The MRC and its researchers benefit by fulfilling their obligation to serve the health needs of the public, thereby enhancing MRC-community relations, and by sharing in any income generated from the invention. Industry benefits through new products that strengthen it and generate wealth. The public benefits from improved quality of life provided by new technologies, products and processes and a strengthened economy. - To publish or to patent?
Publishing an invention allows for its exposure to the scientific community and the rest of the public at an early stage, but does not prevent others from using the information for commercial purposes. Patenting an invention, on the other hand, is extremely expensive and time-consuming, but increases the chances of cutting-edge research discoveries being pursued and developed for the benefit of the public. Patent protection provides a limited monopoly, which is often required by a commercial partner as an incentive to invest in the development of a research discovery into a marketable product.
It is important for inventors to realize, however, that a patent does not guarantee the commercial success of an invention. Many patents fail to generate sufficient income to repay the expense of the patent process. Commercialization is often difficult and it is therefore the job of the IC to assist inventors in assessing the likelihood of economic success before a decision is made to proceed with costly patent protection. - How does filing for a patent affect publications/presentations/posters?
Publishing is one of the most central outputs in the research process. Patent protection does not prevent publishing. It only requires that the publication (including any advance abstract publication) needs to be timed so that it doesn't appear in the Journal or on the web until the appropriate steps are in place to secure the protection of any commercial aspect(s). The journal's publication policy may also need to be considered before disclosing results elsewhere (for example at a conference), as some journals may decline to publish if they are not the first to break the news. A PhD thesis may also be considered to be a disclosure document and patent applications should be filed before the thesis is made publicly available in the relevant institution's library.
Carefully managing the timing of publications is an important aspect of successful technology transfer. Thus, disclosing the idea to the IC as soon as the invention is clearly conceptualized, or at least before submitting abstracts or manuscripts disclosing the invention, allows time to evaluate the invention in terms of patentability and commercialization. If the results of the assessment indicate that the invention is in fact patentable, inventors will usually be advised to delay publication for a defined period.
A significant difference between the United States and the European Union and other patent systems, is the existence of a "grace period" for patent applications in the US. Thus researchers in the US often publish their inventions first and file for a patent later. This essentially means that the inventor forgoes his/her foreign patent rights. - When and why should I carry out a patent search?
Literature and patent searches should be conducted before any research project is initiated in order to avoid duplication of research and intellectual property. Patent searches are particularly important when an invention has been made and needs to be protected. The analysis of "prior art" will enable the preparation of a patent specification which can better anticipate objection, and is therefore more likely to proceed to grant. The other advantage of a patent search is to determine the activities of competitors. - How do I carry out a patent search?
The method used for patent searching depends on the objectives of the search. The most common reason for carrying out a patent search is to determine novelty. Patent searching can be a long, complicated procedure and the results are largely dependent on the keywords used. It is best initially to use broad search terms to ensure that all relevant prior art is identified. Hits must be examined to determine whether they overlap with your invention and to identify other prior art documents that have been identified in the search reports. The MRC IC is able to assist you with patent searching.
Useful websites for patent searching include: - http://www.uspto.gov - US applications and granted patents
- http://www.espacenet.org – Links to US and European Patent Office databases
- http://pctgazette.wipo.int - Published PCT applications
- http://www.ipaustralia.gov.au - Published Australian patents and applications
Sildenafil .........US 20010009962 Patent review
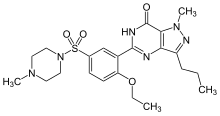

SILDENAFIL
http://www.google.co.ug/patents/US20010009962
This invention relates to a process for the preparation of 1-[[3-(6,7-dihydro-1-methyl-7-oxo-3-propyl-1H-pyrazolo[4,3-d]pyrimidin-5-yl)-4-ethoxyphenyl]sulfonyl]-4-methylpiperazine(otherwise known as sildenafil or Viagra™), and 1-Ethyl-4-{3-[3-ethyl-6,7-dihydro-7-oxo-2-(2-pyridylmethyl)-2H-pyrazolo[4,3-d]pyrimidin-5-yl]-4-propoxyphenylsulphonyl}piperazine and key intermediates thereof.
[0002] 1-[[3-(6,7-dihydro-1-methyl-7-oxo-3-propyl-1H-pyrazolo[4,3-d]pyrimidin-5-yl)-4-ethoxyphenyl]sulfonyl]-4-methylpiperazine (otherwise known as sildenafil) has been found to be particularly useful in the treatment of, inter alia, male erectile dysfunction (WO-A-94/28907), and a process for its preparation is disclosed in EP-A-0463756 (example 12) and Drugs of the Future 1997, 22(2): 138-143. An improved process for preparing sildenafil (over that of EP0463756) is disclosed in EP-A-0812845, with the characterising final step involving cyclisation under basic, neutral or acidic conditions to form sildenafil. A process for the preparation of 1-Ethyl-4-{3-[3-ethyl-6,7-dihydro-7-oxo-2-(2-pyridylmethyl)-2H-pyrazolo[4,3-d]pyrimidin-5-yl]-4-propoxyphenylsulphonyl}piperazine is disclosed in WO 98/49166 (example 5B).
[0003] The present inventors have now found a process for preparing sildenafil and 1-Ethyl-4-{3-[3-ethyl-6,7-dihydro-7-oxo-2-(2-pyridylmethyl)-2H-pyrazolo[4,3-d]pyrimidin-5-yl]-4-propoxyphenylsulphonyl}piperazine which has advantages over the aforementioned prior art processes.
[0004] According to the present invention there is provided a process for preparing a compound of formula (IA) and (IB)
[0005] comprising reacting a compound of (IIA) and (IIB) respectively in the presence of −OR, wherein R in the case of formation of compound (IA) is CH2CH3 and R in the case of formation of compound (IB) is CH2CH2CH3, where X is a leaving group:
[0006] A particular advantage of the present process over that of the prior art is the elimination of steps by carrying out a substitution reaction and ring closure in ‘one pot’.
[0007] The intermediates of general formula (IIA) and (IIB) form a further aspect of the invention.
[0008] A key intermediate of the general formula (IIIA) and (IIIB) (see schemes 1 and 2 hereafter) have been identified in various reactions showing that such reactions at least partially go via a pathway of cyclisation then nucleophilic substitution. Accordingly intermediates of general formula (IIIA) and (IIIB) form yet a further aspect of the invention (wherein X is a leaving group).
[0009] A further major intermediate of formula IVA and IVB have also been identified, suggesting that there is also nucleophilic substitution before cyclisation (and these intermediates, where novel, form a further aspect of the invention).
[0010] Thus the proposed reaction pathways for the formation of compounds (IA) and (IB) are as follows
[0061] (1e) 1-[[3-(6,7-dihydro-1-methyl-7-oxo-3-pronyl-1H-pyrazolo[4,3-d]pyrimidin-5-yl)-4-ethoxyphenyl]sulfonyl]-4-methylpiperazine. (Compound IA)
[0062] Potassium t-butoxide (0.74 g, 6.60 mmol) was added to a suspension of the title compound of example (1d) (1.00 g, 2.20 mmol) in ethanol (5 ml) and the mixture was heated under reflux for 48 hours. The reaction mixture was stripped down to an oil and purified by dissolving in dichloromethane and washing with saturated sodium bicarbonate solution. Hexane was added to the organic solution over 10 minutes, a precipitated solid filtered and dried to afford the title compound (1.1 g, 100%) as a white solid. Recrystallisation of the title compound from ethyl acetate affords a solid with m.p. 184-186° C. Found: C, 55.49; H, 6.35; N, 17.72. C22H31N6O4S requires C, 55.58; H, 6.53; N, 17.68. δ (DMSO): 0.96 (3H, t), 1.30 (3H, t), 1.72 (2H, m), 2.13 (3H, s), 2.36 (4H, m), 2.72 (2H, t), 2.90 (4H, m), 4.18 (5H, m), 7.32 (1H, d), 7.80 (2H, m). m/z (Found: 475.214800 ([M+H]+, 100%). C22H31N6O4S. requires 475.212751).

Spiroindoline derivatives WO2014166958


http://patentscope.wipo.int/search/en/detail.jsf?docId=WO2014166958&recNum=13&maxRec=6164&office=&prevFilter=&sortOption=&queryString=EN_ALL%3Anmr+AND+PA%3Abayer&tab=PCTDescription
The present invention refers to spiroindoline derivatives as gonadotropin-releasing hormone (GnRH) receptor antagonists according to Formula (I)

READ
PATENTWATCH
 SEE A LOVELY SECTION OF ACS PATENTWATCH |
Subscribe to:
Posts (Atom)



