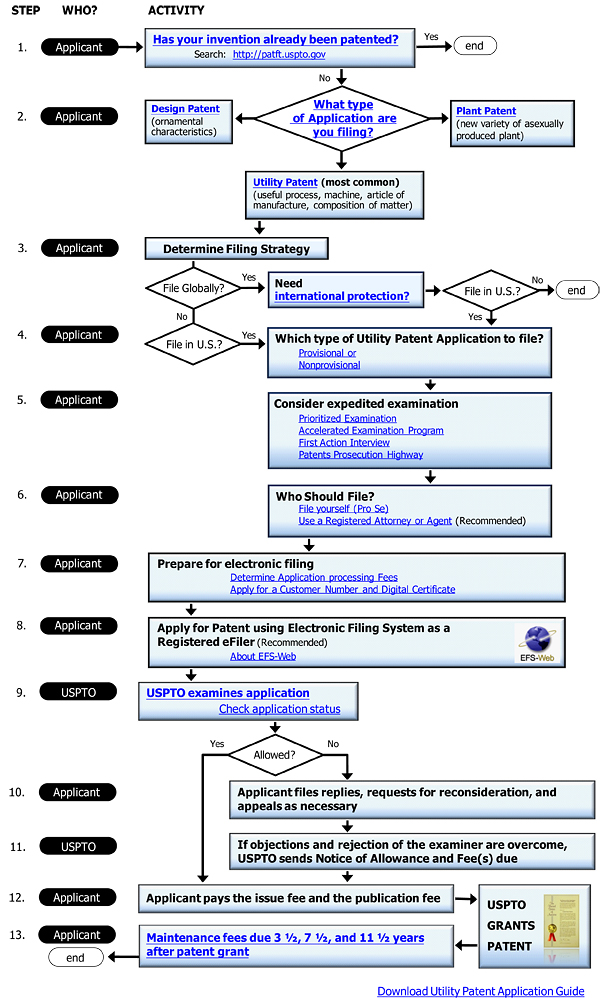
Flowchart of the Process from Application to Grant of a Patent
 INDIA
INDIA
Merits of applying in India
Applying for intellectual property rights in India has the following merits to it.
(1) India is one of the four BRICs countries, attracting attention as a market where high economic growth in the near future is likely.
(2) Because India accepts applications in English, the applicant can use applications in Europe or the United States to apply and thereby save on translation expenses.
(3) Because India is a member of the Paris Convention, the applicant may claim priority when filing a patent granted in Japan.
(4) India is a member of the Patent Cooperation Treaty (PCT) and accepts national phase applications under the PCT.
2. Patents
- 2.1 Duration
Patent rights in India last for twenty years from the application date (India Patent Act Section 53). However, unlike in Japan, there is no system for lengthening the duration of pharmaceutical product inventions, et cetera.
- 2.2 Inventions Subject to Protection
Section 3 of the Indian Patent Act gives specific examples of “things that are not patents.” In India, material patents are acknowledged, but “the mere discovery of a new form of a known substance” (India Patent Act Section 3[d]). Regarding already-known derivatives such as salts, unless they substantially differ in efficacy from known substances, they are considered equivalent to the known substances.
Software itself will not be awarded a patent, but software that is combined with some form of hardware may pass.
India does not have a utility model system.
- 2.3 Application
Figure 1 shows the flow of a patent application from the opening of an application to registration of a patent. Here follows a simple explanation of each stage.
- Application -
Because India is a member of the Paris Convention, the applicant may claim priority based on a Japanese patent application.
Because India is a member of the PCT, the applicant may specify India in a PCT international application.
Within six months of application, the applicant must submit information on foreign applications (India Patent Act Section 8 [1], India Patent Rules 12 [1A]). If before the grant or rejection of grant of a patent, the Controller requests particular details concerning the processing of the application in other countries, the applicant must submit such details (India Patent Act Section 8 [2], Patent Rules 12 [3]).
- Publication of the Application -
An application is published eighteen months from either the date of application or the priority date of the relevant application (India Patent Rules 24). If an applicant wishes to acquire patent rights early, he or she should utilize the early publication system (India Patent Act Section 11 [2]).
- Request for Examination -
The applicant must make a request for examination within forty-eight months of the priority date of the relevant application or of the application date (India Patent Rules 24B). If the request is not made within this period, the India Patent Office will treat the application as if it had been withdrawn (India Patent Act Section 11 [B]).
- Examination -
If the examiner finds any objections to granting a patent, a First Examination Report is issued. If the applicant responds to this office action, the examiner will examine the application again. If no reason is found for rejection, a decision to grant the patent is made.
If the applicant does not revise the application so that grant of a patent is possible within twelve months (the Acceptance Period) after the First Examination Report is sent, the application will be treated as abandoned (India Patent Act Section 21 [1], India Patent Rules 24 [B]).
If an application is rejected, the applicant may file a request for re-examination (under India Patent Act Section 77 [f]) or appeal (under India Patent Act Section 117A).
(Procedures that the Applicant May Take during the Examination Period)
(a) Amendment
With permission from the Controller, the applicant may amend the application (India Patent Act Section 57). However, the Controller will not recognize an amendment when the amended specification claims or describes things that were not substantially disclosed or indicated in the specification prior to the amendment, or when the claims of the amended specification will not completely fit within the parameters of the pre-amendment specification’s claims (India Patent Act Section 59).
(b) Divisional Application
If a patent has not yet been granted, the applicant may divide the application at any time (India Patent Act Section 16).
(c) Interview with the Examiner
The applicant may seek to have an interview with the examiner after the examiner has begun to examine the application. According to representatives in India, an interview with the examiner is an extremely effective measure.
- Opposition to Grant of a Patent Prior to the Granting of the Patent -
After the publication of a patent application, if a patent has not been granted, anyone may register an opposition to the patent grant with the Controller (India Patent Act Section 25 [1]).
- 2.4 After Grant of a Patent
Following the grant of a patent and up to one year after the announcement of the grant of the patent, anyone privy to the matter may register an opposition to the grant (India Patent Act Section 25 [2]).
Apart from an opposition to the grant of a patent, one may file an application to have the patent revoked (India Patent Act Section 64).
The patentee must give a working statement regarding exploitation of the patent annually (Indian Patent Act Section 146, India Patent Rules 131 [2]).
A patented invention that has not been exploited in India’s territory within three years after the granting of the patent may be cause for grant of a compulsory license (India Patent Act Section 84).
If two years have passed since the order for the first licensing of a compulsory license and the patented invention is still not being exploited in India’s territory, the patent may be revoked (India Patent Act Section 85).
3. Designs
- 3-1. Duration
The duration of a design is ten years from its registration date and may be extended only once for five more years (India Designs Act Section 11). Note that the registration date is treated as the date of application for registration (India Design Act Section 5 [6]).
- 3.2 Designs Subject to Protection
Section 2(d) of the India Designs Act defines design: “‘design’ means only the features of shape, configuration, pattern, ornament or composition of lines or colours applied to any article . . . by any industrial process or means . . .”
Section 2(a) of the India Designs Act defines article: “‘article’ means any article of manufacture and any substance, artificial, or partly artificial and partly natural and includes any part of an article capable of being made and sold separately.” Furthermore, “partial designs” which cannot be sold independently of a whole design are not protected.
- 3.3 Application
Because India is a member of the Paris Convention, the applicant may claim priority based on a Japanese right.
A request for examination is not necessary (India Designs Act Section 5).
If the examination results in a rejection decision, the applicant may appeal to the High Court (India Designs Act Section 5 [4]).
- 3.4 After Grant of a Design
Anyone privy to the matter may file an application to have the design right revoked (India Designs Act Section 19).
4. Trademarks
- 4.1 Duration
Trademarks are protected for ten years. Trademark protection may be extended with an application for renewal.
- 4.2 Trademarks Subject to Protection
In addition to product trademarks, service marks are protected (India Trademark Act Section 2 [1] [zb]).
There is a system for organizational trademarks (Section 61).
Three-dimensional shapes and color combinations are also protected (Section 2 [1] [m]).
- 4.3 Application
Because India is a member of the Paris Convention, the applicant may claim priority based on a Japanese right. However, India is not a member of the Madrid Protocol.
As in Japan, India follows the one application, one trademark policy, but within one application the applicant may specify multiple sections (India Trademark Act Section 18).
The applicant may pay an additional fee to request an early examination (India Trademark Rules Section 38 [1]).
If the result of the examination is a rejection decision, the applicant may make an appeal (under India Trademark Act Section 91).
India has a system for challenging trademarks, and for the three months after the trademark is announced, anyone may register an objection to the trademark grant (India Trademark Act Section 25 [1]).
- 4.4 After Registration
In renewal examinations, whether or not the trademark is actually being used is not judged.
Anyone privy to the matter may request a trial for invalidation of a trademark (India Trademark Act Section 57).
If a trademark is not used for five years and three months, it may be subject to a trial for cancellation for non-use (India Trademark Act Section 47).
When the agent or representative of an owner of the a registered trademark files a registration or other paperwork in his or her own name without receiving a power of attorney, the owner of the trademark may register an objection against this paperwork (India Trademark Act Section 146). However, the owner must do so within three years of knowing of the problem.
[Bibliography]
1. International Association for the Protection of Intellectual Property (AIPPI) Japan. Investigation report on India’s intellectual property protection systems and their operation statuses. 2007.
2. “The influence of India’s Patent Act revisions.” In Patento 61, No. 2 (2008), 42-48.
3. Third International Committee. Points to remember when acquiring patents in countries of Asia and Oceania. Revised edition. Resource No. 332. May 2006.