Processes for the preparation of lorcaserin
Zydus Cadila Healthcare Ltd
WO 2015102017, 09 July2015

On 10 May 2012, after a new round of studies submitted by Arena, an
FDA panel voted to recommend lorcaserin with certain restrictions and
patient monitoring. The restrictions include patients with a
BMI of over 30, or with a BMI over 27 and a comorbidity such as high blood pressure or type 2 diabetes.
On 27 June 2012, the FDA officially approved lorcaserin for use in the treatment of obesity for adults with a
BMI equal
to or greater than 30 or adults with a BMI of 27 or greater who “have
at least one weight-related health condition, such as high blood
pressure, type 2 diabetes, or high cholesterol
Useful for treating obesity.
The present invention relates to stable crystalline Form I of
Iorcaserin hydrochloride of Formula (IA) and processes for its
preparation. The invention also relates to processes for the preparation
of lorcaserin and pharmaceutically acceptable salts, solvates and
hydrates thereof.
Stable crystalline form I of lorcaserin hydrochloride and its process
of preparation are claimed. Represents the first patenting from Cadila
on lorcaserin, which was developed and launched by Arena Pharma and
Eisai.
In July 2015, Newport Premium™ reported that Cadila is potentially interested in lorcaserin.
Lorcaserin hydrochloride is an agonist of the 5-HT
2c receptor
and shows effectiveness at reducing obesity in animal models and humans
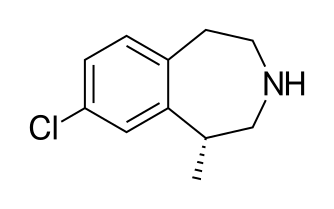
developed by Arena Pharmaceuticals. It is chemically represented as
(R)-8-chloro-l -methyl -2,3,4,5-tetrahydro-lH-3-benzazepine
hydrochloride having Formula (I) as depicted herein below.

(IA)
U.S. Patent No. 6,953,787 B2 discloses compound of Formula (I) and
pharmaceutically acceptable salt, solvates or hydrates thereof and
process for preparation thereof.
U.S. Patent No. 8,168,624 B2 discloses
(R)-8-chloro-l-methyl-2,3,4,5-tetrahydro-lH-3-benzazepine hydrochloride
hemihydrate and process for its preparation. The patent also discloses
crystalline Form I, Form II and Form III of
(R)-8-chloro-l-methyl-2,3,4,5-tetrahydro-lH-3-benzazepine hydrochloride.
The crystalline Form
I and Form II are reported as anhydrous, non-solvated crystal forms.
The crystalline Form III displays a dehydration feature calculated as a
3.7% weight loss which is consistent with the theoretical weight loss of
3.7% for a hemihydrate.
The patent discloses that anhydrous Form I and Form II readily
converts to a hemihydrate, upon exposure to moisture. The dynamic vapor
sorption (DVS) data for each of the three crystal forms reveals the
hygroscopic nature of both Forms I and II, which readily adsorb moisture
at relative humidity (RH) greater than about 40-60%. In addition, both
Forms I and II were calculated to adsorb about 3.8% moisture between
about 40 and about 80% RH which is consistent with conversion to the
hemihydrate (Form III). X-ray powder diffraction (XRPD) carried out on
both Forms I and II after the DVS cycle confirmed this conversion. In
contrast, the DVS data in connection with Form III shows that it is
substantially non-hygroscopic, adsorbing less than 0.5% water at 90% RH
and the XRPD pattern showed no change in crystalline form after the DVS
cycle.
International (PCT) Publication Nos. WO 2003/086306 Al, WO
2005/019179 Al, WO 2006/069363 Al, WO 2007/120517 Al, WO 2008/07011 1 Al
and WO 2009/1 1 1004 Al disclose various synthetic approaches for the
preparation of
(R)-8-chloro-l-methyl-2,3,4,5-tetrahydro-lH-3-benzazepine, its related
salts, enantiomers, crystalline forms and intermediates.
International (PCT) Publication No. WO 2006/071740 Al discloses
combination of (R)-8-chloro-l-methyl-2,3,4,5-tetrahydro-lH-3-benzazepine
with other agents. International (PCT) Publication No. WO 2012/030938
Al discloses various salts of lorcaserin with optically active acids.
U.S. PG-Pub No. US 2014/0187538 Al discloses amorphous lorcaserin
hydrochloride and amorphous solid dispersion comprising lorcaserin
hydrochloride and one or more pharmaceutically acceptable carriers and
processes for their preparation.
International (PCT) Publication No. WO 2014/135545 Al discloses solid
dispersion comprising amorphous lorcaserin hydrochloride and one or
more pharmaceutically acceptable water soluble polymers.
see…..h
ttps://patentscope.wipo.int/search/en/detail.jsf?docId=WO2015102017&recNum=1&maxRec=&office=&prevFilter=&sortOption=&queryString=&tab=PCTDescription

Example-7: Preparation of crystalline Form I of lorcaserin
hydrochloride. In a round bottom flask, 560g of methyl ethyl ketone and
40 ml water were taken and 100 g of
8-chloro-l-methyl-2,3,4,5-tetrahydro-lH-3-benzazepine was added and
stirred for 10 minutes. The reaction mass heated to 55 to 60°C and 19.3 g
of. L-(+)-tartaric acid was added slowly and stirred for one to two
hours. The reaction mass was further stirred at 10-15°C for an hour and
the product was filtered and washed with a mixture of methyl ethyl
ketone and water. The wet cake and 150 ml methyl ethyl ketone were taken
in another flask and heated to 75-80°C. 20-25 ml water was, added and
stirred for an hour. Further, the reaction mass was stirred for an hour
at 0-5°C. The product was filtered and washed with methyl ethyl ketone.
100 g tartrate salt of
8-chloro-l-methyl-2,3,4,5-tetrahydro-lH-3-benzazepine and 300 mL water
were taken in another round bottom flask. 200 mL methylene dichloride
was added and the reaction mass was cooled to 10-20°C. 17.2 g sodium
hydroxide dissolved in 89 ml water was added into the reaction mass at
10-20°C. The reaction mass was stirred for an hour at 25-30°C and the
layers were separated. The solvent was removed from the organic layer
under vacuum and then 100 mL ethyl acetate was added into that and
distilled out. Further, 100 mL ethyl acetate was added and stirred for
15 minutes. The reaction mass was filtered through a hyflow bed and the
filtrate was treated with dry HC1 gas till a pH of 1.5 to 2.5 was
obtained at 0-10°C and it was stirred for about 30 minutes to an hour.
The product was then filtered and washed with ethyl acetate and then
dried in a vacuum oven at 50°C to 55°C for 2 hours. The product was
further dried at 90°C to 110°C for 20 hours to obtain crystalline Form I
of lorcaserin hydrochloride. Yield: 87.5-98.6 %.
Example-8: Preparation of crystalline Form I of lorcaserin hydrochloride

In a round bottom flask, 2.20 g lorcaserin, 30 mL methylene chloride,
17.4 mL of 1M HCI in ether were added and the mixture was stirred for
5-15 minutes at room temperature. The solvent was removed under reduced
pressure to give a white solid. This solid was again dissolved in 30 ml
methylene chloride, 17.4 mL of 1M HCI solution and stirred for 5-15
minutes at room temperature. The solvent was removed under reduced
pressure to give lorcaserin hydrochloride. The product was dried in a
vacuum oven at 50°C to 55°C for 2 hours. The product was further dried
at 90°C to 110°C for 20 hours to obtain crystalline Form I of lorcaserin
hydrochloride.
Example-9: Preparation of crystalline Form I lorcaserin hydrochloride
50 g of lorcaserin hydrochloride hemihydrate and 50 g of
hydroxypropylmethyl cellulose (HPMC) 3CPC were mixed in a blender at
25°C to 35°C. The mixture was mixed for 30 minutes and unloaded. The
solid thus obtained was dried in a vacuum oven at 50°C to 55°C for 2
hours. The product was further dried at 90°C to 110°C for 20 hours to
obtain crystalline Form I of lorcaserin hydrochloride.

Pankaj R. Patel (right), Chairman and Managing Director,
/////////




































 LIONEL MY SON
LIONEL MY SON