WO2016181313, A PROCESS FOR THE PREPARATION OF SOFOSBUVIR INTERMEDIATES & ITS POLYMORPH
| LUPIN LIMITED [IN/IN]; Kalpataru Inspire 3rd Floor, Off Western Express Highway Santacruz (East) Mumbai 400 055 (IN) |
SINGH, Girij, Pal; (IN).
SRIVASTAVA, Dhananjai; (IN).
MEHARE, Kishor, Gulabrao; (IN).
MALIK, Vineet; (IN).
DEOKAR, Sharad, Chandrabhan; (IN).
DANGE, Abhijeet, Avinash; (IN)
SRIVASTAVA, Dhananjai; (IN).
MEHARE, Kishor, Gulabrao; (IN).
MALIK, Vineet; (IN).
DEOKAR, Sharad, Chandrabhan; (IN).
DANGE, Abhijeet, Avinash; (IN)


SUCCESS QUOTIENT: Lupin chairman DB Gupta (sitting) with managing director Kamal K Sharma (centre), directors Vinita Gupta (right) and Nilesh Gupta.
The present invention provides a novel process for preparation N-[(2,3,4,5,6- Pentafluorophenoxy)phenoxyphosphinyl]-L-alanine 1-methylethyl ester (formula 2) and resolving the formula 2 in the presence base to form N-[(S)-(2,3,4,5,6- Pentafluorophenoxy)phenoxyphosphinyl]-L-alanine 1-methylethyl ester (formula 2').
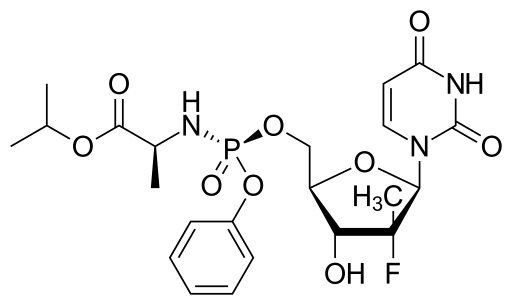
Sofosbuvir is chemically named as (S)-isopropyl 2-((S)-(((2R,3R,4R,5R)-5-(2,4- dioxo3,4-dihydropyrimidin-l(2H)-yl)-4-fluoro-3-hydroxy-4-methyltetrahydrofuran- 2yl)methoxy)-(phenoxy)phosphorylamino)propanoate and is represented by the following chemical structure:

Formula 1
PCT publications WO2011123645 and WO2010135569 describes process for preparation of compound of formula 2' by reacting isopropyl (chloro(phenoxy)phosphoryl)-L-alaninate and pentaflurophenol in the presence of base.

Formula 2'
Example-1:
Preparation of sodium 2,3,4,5,6-pentaflurophenolate using sodium hydride
10.2g of sodium hydride was dissolved in 100 ml anhydrous THF. This solution was slowly added to a solution of pentafluorophenol (50g) in THF (100ml), Reaction mass was stirred for 60-120 min at 25-30°C. Reaction mass was distilled under reduced pressure, obtained solid was dried under vacuum at 45-50°C (yield=55g, confirmed by IR)
Example-2:
Preparation of sodium 2,3,4,5,6-pentaflurophenolate using sodium methoxide
2,3,4,5, 6-pentafluorophenol (lOg) was dissolved in methanol (100ml), solution was cooled to 5-10°C. To this was added a solution of sodium methoxide in methanol. The reaction mass was stirred for 60-120 min at 25-30°C. Reaction mass was distilled under reduced pressure, obtained residue was striped with toluene. Obtained solid was dried under vacuum at 45-50°C (yield=l lg)
Example 3:
Preparation of sodium 2,3,4,5,6-pentaflurophenolate using sodium hydroxide
2,3,4,5, 6-pentafluorophenol (lOOg) dissolved in methanol (—ml), solution was cooled to 5-10°C. To this was added a solution of sodium hydroxide (— g) in methanol. The reaction mass was stirred for 60-120 min at 25-30°C. Reaction mass was distilled under reduced pressure, obtained residue was striped with dichloromethane. Obtained solid was dried under vacuum at 45-50°C (yield=— g)
Example 4:
Preparation of (2S)-isopropyl-2-((chloro(phenoxy)posphoryl)amino)propanoate:
phenyl phosphodichloridate (30.6g) was dissolved in dichloromethane , to this was added a solution of 1-alanine isopropyl ester free base (19.16g) in dichloromethane at-60°C under nitrogen. Solution of triethylamine (20.7ml) was added to above reaction mass. Reaction mass was stirredat -60°C for 30 min and then temperature was raised to 25 °C. Reaction mass was stirred at 20-25 °C for 60 min & filtered and washed with dichloromethane. Clear filtrate was distilled under reduced pressure obtained residue was stirred with diisopropyl ether & filtered. Clear filtrate was distilled under reduced pressure to get (2S)-isopropyl-2-((chloro(phenoxy)posphoryl)amino)propanoate compound.
Example 5:
Preparation of isopropyl ((perfluorophenoxy)(phenoxy)phosphoryl)-L-alaninate (formula 2):

(Formula 2)
Obtained (2S)-isopropyl-2-((chloro(phenoxy)phosphoryl)amino)propanoate (1.2 mol eq.) was dissolved in dichloromethane and cooled to 0-5°C under nitrogen atmosphere. To this was added solution of sodium 2,3,4,5,6-pentaflurophemolate (1 mol eq.) in tetrahydrofuran . Temperature of reaction mass was raised to 25°C and reaction mass was stirred for 3 hrs. After completion of reaction, reaction mass was distilled under reduced pressure & obtained residue was dissolved I ethyl acetate. Ethyl acetate layer was washed with water, dried over sodium sulfate & distilled off under reduced pressure. Diisopropyl ether was added to obtained residue and stirred for 60 min at 25 °C, obtained mass was filtered & washed with diisopropyl ether. Solid product was dried under vacuum at 40-45 °C .(yield=20g, enantiomer purity=93.45%)
Example 6:
Preparation of (S)-isopropyl 2-(((S)- (perfluorophenoxy)phenoxy)phosphoyl)amino)propanoate (Formula 2'):

Formula 2'
(2S)-isopropyl-2-((chloro(phenoxy)phosphoryl)amino)propanoate (1.2 mol eq.) was dissolved in tetrahydrofuran (3.5 volumes). The reaction mass was cooled to -10°C. Solution of sodium salt of pentafluorophenol (1 mol eq.) in tetrahydrofuran (3.5 volumes) was added dropwise to the reaction mass at -10°C. After completion of the reaction solvent was distilled off. Ethyl acetate and water were added to the reaction mass. Reaction mass was stirred, ethyl acetate layer was separated and washed with sodium bicarbonate solution and brine. Ethyl acetate layer was concentrated under reduced pressure. Reaction mass was stripped with n-hepatane to get crude product. Crude product was dissolved in Methyl tert-butyl ether and n-heptane (1 : 1 ratio). The pH of reaction mass was adjusted to pH 8 by using triethylamine. Reaction mass was stirred overnight. Solid mass was filtered and washed with a mixture of methyl tertiary-butyl ether: n-heptane (1 : 1). The obtained product was dissolved in ethyl-acetate and washed with water and 20% brine solution. Ethyl acetate layer was separated; solvent was distilled off under reduced pressure. Reaction mass was stripped with diisopropyl ether. Di-isopropyl ether was added to the reaction mass. Reaction mass was stirred at 45-50°C. Reaction mass was cooled to 5-10°C and stirred. The titled compound was isolated by filtration and washed with di-isopropyl ether. The titled compound was dried under reduced pressure at 40°C. Yield 66.81%.

Vinita Gupta, CEO, Lupin Pharmaceuticals Inc
Desh Bhandu Gupta- Founder and chairman of Lupin Limited
////////////LUPIN LIMITED, WO 2016181313, NEW PATENT, SOFOSBUVIR