WO2016012938, IMPROVED PROCESS FOR PREPARATION OF AMORPHOUS LINACLOTIDE
DR. REDDY’S LABORATORIES LIMITED [IN/IN]; 8-2-337, Road No 3, Banjara Hills, Telangana, INDIA Hyderabad 500034 (IN)
KALITA, Dipak; (IN).
NIVRUTTI, Ramrao Jogdand; (IN).
BALAKUMARAN, Kesavan; (IN).
DESHMUKH, Shivshankar; (IN).
VUTUKURU, Naga Chandra Sekhar; (IN).
KASINA, Vara Prasad; (IN).
NALAMOTHU, Sivannarayana; (IN).
VILVA, Mohan Sundaram; (IN).
KHAN, Rashid Abdul Rehman; (IN).
TIRUMALAREDDY, Ramreddy; (IN).
MUSTOORI, Sairam; (IN)
NIVRUTTI, Ramrao Jogdand; (IN).
BALAKUMARAN, Kesavan; (IN).
DESHMUKH, Shivshankar; (IN).
VUTUKURU, Naga Chandra Sekhar; (IN).
KASINA, Vara Prasad; (IN).
NALAMOTHU, Sivannarayana; (IN).
VILVA, Mohan Sundaram; (IN).
KHAN, Rashid Abdul Rehman; (IN).
TIRUMALAREDDY, Ramreddy; (IN).
MUSTOORI, Sairam; (IN)

The present application relates to an improved process for the formation of disulfide bonds in linaclotide. The present application also relates to an improved process for the purification of linaclotide.
The present application relates to an improved process for the preparation of amorphous linaclotide. Specifically, the present application relates to an improved process for the formation of disulfide bonds in linaclotide. The present application further relates to a purification process for the preparation of amorphous linaclotide.
INTRODUCTION
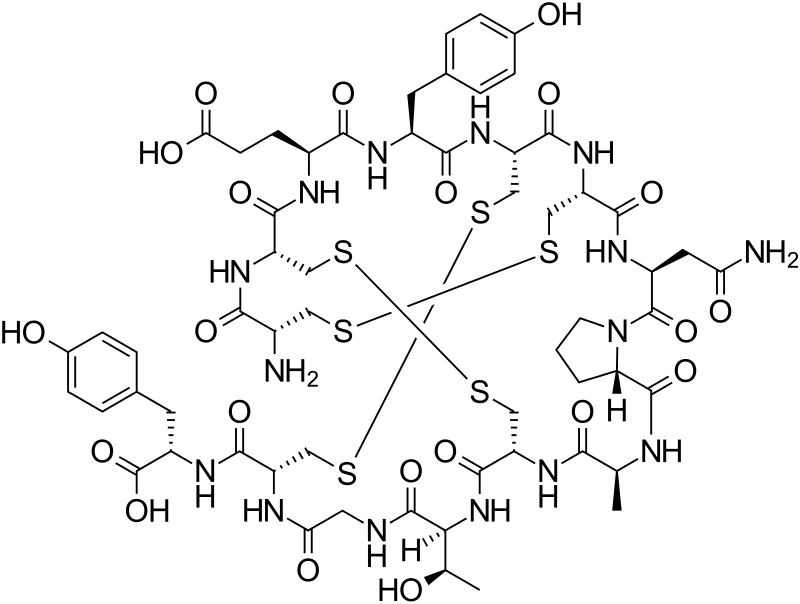
Linaclotide is a 14-residue peptide which is an agonist of the guanylate cyclase type-C receptor. Linaclotide may be used for the treatment of chronic constipation and irritable bowel syndrome. Structurally, linaclotide has three disulfide bonds and they are present between Cys1-Cys6, Cys2-Cys-10 and Cys5-Cys13. The structure of linaclotide is shown below:
1 2 3 4 5 6 7 8- 9 10 11 12 13 14

Benitez et al. Peptide Science, 2010, Vol. 96, No. 1 , 69-80 discloses a process for the preparation of linaclotide. The process involves the use of 2-chlorotrityl (CTC) resin and 9-fluorenylmethoxycarbonyl (Fmoc) chemistry. The Cys residues are protected by Trt (trityl) group. The amino acids are coupled to one another using 3 equivalents of 1 -[bis(dimethylamino)methylene]-6-chloro-1 H-benzotriazolium hexafluorophosphate 3-oxide (HCTU) as coupling agent and 6 equivalents of diisoprpylethylamine (DIEA) as base in dimethylformamide (DMF). The Fmoc group is removed using piperidine-DMF (1 :4). The Cys residues are incorporated using 3 equivalents of Ν,Ν'-diisopropylcarbodiimide (DIPCDI) as coupling agent and 3 equivalents of 1 -hydroxybenzotriazole (HOBt) as an activating agent. After the elongation of the peptide chain, the peptide was cleaved from the solid support (CTC resin) by first treating with 1 % trifluoroacetic acid (TFA) and then with a mixture of TFA, triisoprpylsilane (TIS) and water in the ratio of 95:2.5:2.5. The disulfide bonds are prepared by subjecting the linear peptide to air oxidation in sodium dihydrogen phosphate (100 mM) and guanidine hydrochloride buffer (2 mM).
US2010/261877A1 discloses a process for purification of linaclotide. The process involves first purification of crude peptide by reverse-phase chromatographic purification followed by concentrating the purified pools and dissolving the purified linaclotide in aqueous-isopropanol or aqueous-ethanol and spray-drying the solution to afford pure Linaclotide.
The synthesis of a peptide containing disulfide bridges is difficult for two main reasons; one is potential risk of racemization during the formation of linear chain and the other is mis-folding of the disulfide bridges. Hence, there is a need in the art to a cost-effective process for the preparation of pure linaclotide.
EXAMPLES
Example 1 : Preparation of Crude Linaclotide using polyvinyl polymer bound complex of sulfur trioxide-pyridine
The linear chain of peptide of formula (I) (0.1 g) and polyvinyl polymer bound complex of sulfur trioxide-pyridine (0.062 g) was charged in water (100 mL). The pH of the reaction mass was adjusted to 8.5 to 9 by addition of ammonium hydroxide. The reaction mass was stirred at 25 °C for 15 hours and trifluoroacetic acid (2 mL) was added to the reaction mass to adjust the pH up to 2-2.5. The reaction mass was stirred for 3 hours at the same temperature to afford crude linaclotide.
HPLC Purity: 59.92%
Example 2: Preparation of Crude Linaclotide using DMSO in water
The pH of water (100 ml_) was adjusted to 9.1 by the addition of aqueous ammonia. DMSO (1 ml_) and linear chain of peptide of formula (I) (100 mg) were charged. The reaction mass was stirred for 17 hours at 25 °C and acidified with trifluoroacetic acid to pH 1 .9 and stirred for 8 hours at the same temperature to afford crude linaclotide.
HPLC Purity: 57%
Example 3: Preparation of Crude Linaclotide using DMSO in water
The pH of water (1500 ml_) was adjusted to 9 by the addition of aqueous ammonia. DMSO (15 ml_) and linear chain of peptide of formula (I) (15 g) were charged. The reaction mass was stirred for 17 hours at 25 °C and acidified with acetic acid to pH 1 .9 and stirred for 8 hours at the same temperature to obtain crude linaclotide.
HPLC Purity: 46.02%
Example 4: Preparation of Crude Linaclotide in water
To a mixture of water (1900 mL) and ammonium sulfate (26.4 g), ammonium hydroxide was added drop wise to adjust the pH up to 8.5. Linear chain of peptide of formula (I) (26.4 g) was added and the reaction mass was stirred for 8 hours at 25 °C. Trifluoroacetic acid (20 mL) was added drop wise and the reaction mixture was stirred for 15 hours at 25 °C to afford crude linaclotide.
HPLC Purity: 63.38%
Example 5: Preparation of Crude Linaclotide using a complex of pyridine-sulfur trioxide
Linear chain of peptide of formula (I) (0.2 g) was added to water (250 mL) and the pH of the reaction mass was adjusted to 8.91 by the drop wise addition of aqueous ammonia. A complex of pyridine-sulfur trioxide (0.124 g) was added to the reaction mass and stirred for 16 hours at 25 °C. Another lot of complex of pyridine-sulfur trioxide (0.124 g) was added to the reaction mass and stirred for 5 hours at 25 °C to afford crude linaclotide.
Example 6: Preparation of Crude Linaclotide using guanidine hydrochloride
To a solution of sodium bicarbonate (0.89 g) in water (100 mL), cysteine (0.363 g), cysteine (0.072 g) and guanidine hydrochloride (9.50 g) were charged. Acetonitrile (15 mL) and linear chain of peptide of formula (I) (0.1 g) was added to the reaction mass.
The reaction mass was stirred for 3 hours at 25 °C and trifluoroacetic acid (2 mL) was added. The reaction mass was stirred for 18 hours at the same temperature. Another lot of trifluoroacetic acid (2 mL) was added to the reaction mass and stirred for 18 hours at the same temperature to afford crude linaclotide.
Example 7: Preparation of Crude Linaclotide using Clear-OX™
Pre-conditioned Clear-Ox™ (0.5 g) was added to a solution of ammonium sulfate (1 .32 g) in water (100 mL) of pH 8.5, adjusted by addition of ammonium hydroxide. The linear chain of peptide of formula (I) (0.1 g) was added to the reaction mass and stirred for 3 hours at 25 °C. Another lot of Pre-conditioned Clear-Ox™ (0.5 g) was added to the reaction mass and stirred for 1 .30 hours. Trifluoroacetic acid (2 mL) was added to the reaction mass and stirred for 16 hours at the same temperature to afford crude linaclotide.
HPLC Purity: 67.5%
Example 8: Preparation of Crude Linaclotide using reduced Glutathione
To a mixture of ammonium sulphate (5.28 g) in water (400 mL) and isopropyl alcohol (400 mL), reduced glutathione (0.248 g) was added and the pH was adjusted to 8.5 by using aqueous ammonia. The linear chain of peptide of formula (I) (0.81 g) was added to the reaction mixture and stirred at ambient temperature for 17 hours. Isopropyl alcohol was evaporated under vacuum to afford crude linaclotide.
HPLC Purity: 69.56%%
Example 9: Preparation of Crude Linaclotide using DMSO and air bubbling
To a mixture of water (95 mL) and ammonium sulfate (1 .32 g), ammonium hydroxide was added drop wise to adjust the pH up to 8.5. Linear chain of peptide of formula (I) (0.1 g) and DMSO (5 mL) was added and the reaction mass was stirred for 20 hours at 25 °C with continuous air bubbling. Trifluoroacetic acid (2 mL) was added to the reaction mass and stirred for 19 hours with continuous air bubbling at the same temperature to afford the title product.
HPLC Purity: 59.1 1 %
Example 10: Preparation of Crude Linaclotide using solid supported TEMPO
To a mixture of water (100 mL) and silica bound TEMPO (0.01 g), linear chain of peptide of formula (I) (0.1 g) and sodium hypochlorite solution (1 mL) were added and the reaction mass was stirred 18 hours at 25 °C. Another lot of sodium hypochlorite solution (0.5 mL) was added to the reaction mass and stirred for further 7 hours at the same temperature to afford title product.
HPLC Purity: 42.70%..................see more in patent
 | |
| Systematic (IUPAC) name | |
|---|---|
| L-Cysteinyl-L-cysteinyl-L-glutamyl-L-tyrosyl-L-cysteinyl-L-cysteinyl-L-asparaginyl-L-prolyl-L-alanyl-L-cysteinyl-L-threonylglycyl-L-cysteinyl-L-tyrosine cyclo(1-6),(2-10),(5-13)-tris(disulfide) | |
| Clinical data | |
| Trade names | Linzess |
| Licence data | US FDA:link |
| Pregnancy category |
|
| Legal status |
|
| Routes of administration | Oral |
| Identifiers | |
| CAS Number | 851199-59-2 |
| ATC code | A06AX04 |
| PubChem | CID 16158208 |
| IUPHAR/BPS | 5017 |
| ChemSpider | 17314504 |
| UNII | N0TXR0XR5X |
| KEGG | D09355 |
| Chemical data | |
| Formula | C59H79N15O21S6 |
| Molar mass | 1526.74 g/mol |
///////WO 2016012938, DR. REDDY’S LABORATORIES LIMITED , Telangana, INDIA , Hyderabad, LINACLOTIDE, new patent
smiles O=C(O)[C@@H](NC(=O)[C@H]4NC(=O)CNC(=O)[C@@H](NC(=O)[C@H]2NC(=O)[C@@H](NC(=O)[C@H]5N(C(=O)[C@@H](NC(=O)[C@H]1NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CSSC1)CSSC2)CCC(=O)O)Cc3ccc(O)cc3)CSSC4)CC(=O)N)CCC5)C)[C@H](O)C)Cc6ccc(O)cc6