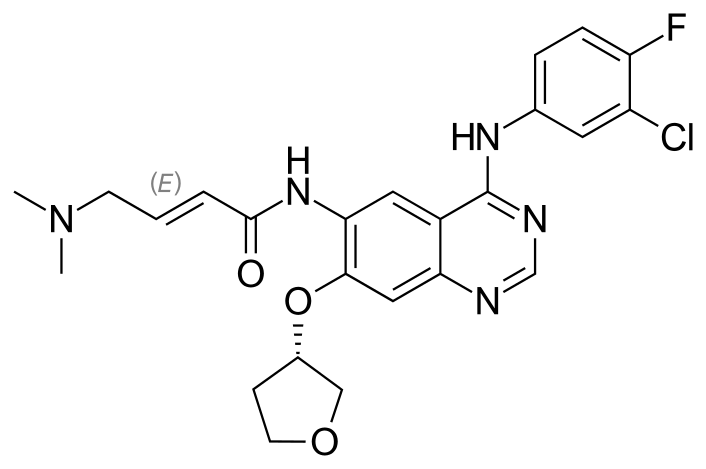
Afatinib
439081-18-2
850140-73-7 dimaleate
Tovok, BIBW2992, Tomtovok
An irreversible EGFR/HER2 inhibitor
| Molecular Weight: | 485.94 |
| Molecular Formula: | C24H25ClFN5O3 |
N-[4-[(3-Chloro-4-fluorophenyl)amino]-7-[[(3S)-tetrahydro-3-furanyl]oxy]-6-quinazolinyl]-4(dimethylamino)-2-butenamide
4 - [(3-chloro-4-fluorophenyl) amino] -6 - {[4 - (N, N-dimethylamino)-1-oxo-2-buten-1-yl] - amino} -7 - ((S )-tetrahydrofuran-3-yloxy)-quinazoline
(E)-4-Dimethylamino-but-2-enoic acid {4-(3-chloro-4-fluoro- phenylanimo)-7-[(S)-(tetrahydro-furan-3-yl) oxy]-quinazolin-6-yl} -amide
4 - [(3_ chloro-4 - fluorophenyl) amino] -6 - {[4_ (N, N-dimethylamino)-buten-1-oxo-_2_ - yl] amino}-7 - ((S) - tetrahydrofuran-3 - yloxy) - quinazoline
Sun Pharmaceutical Industries Ltd.
Pharmaceutical Company
Address: Sun House, CTS No. 201 B/1, Western Express Highway, Goregaon East, Mumbai, Maharashtra 400063
PATENT
PROCESS FOR THE PREPARATION OF 4-DIMETHYLAMINOCROTONIC ACID
SUN PHARMACEUTICAL INDUSTRIES LIMITED [IN/IN]; Sun House, Plot No. 201 B/1 Western Express Highway Goregaon (E) Mumbai, Maharashtra 400 063 (IN)
VERMA, Shyam Sunder; (IN).
SINGH, Shravan Kumar; (IN).
SINGH, Kaptan; (IN).
PRASAD, Mohan; (IN)
SINGH, Shravan Kumar; (IN).
SINGH, Kaptan; (IN).
PRASAD, Mohan; (IN)
Afatinib is a tyrosine kinase inhibitor disclosed in U.S. Patent Nos. RE43,431 and
6,251,912. Afatinib is depicted by Formula la:

Formula la
Afatinib is presented as the dimaleate salt and is chemically designated as 2-butenamide, N-[4-[(3-chloro-4-fluorophenyl)amino]-7-[[(35)-tetrahydro-3-furanyl]oxy]-6-quinazolinyl]-4-(dimethylamino)-,(2£)-,(2Z)-2-butenedioate (1 :2) having the structure depicted by Formula I:

Formula I
Processes for the preparation of 4-dimethylaminocrotonic acid or its salts are disclosed in U.S. Patent No. 7,126,025 and U.S. Publication No. 2012/0046494.
U.S. Patent No. 7,126,025 discloses a process for the preparation of 4-dimethylaminocrotonic acid or its salts by reacting but-2-enoic acid with
chlorotrimethylsilane in pyridine to obtain trimethylsilylcrotonate, which is brominated with a brominating agent under free radical conditions and in the presence of methylene chloride, acetonitrile, 1,2-dichloroethane, carbon tetrachloride, or ethyl acetate to give trimethylsilyl-4-bromocrotonate. The bromocrotonate compound is treated with dimethylamine in tetrahydrofuran to provide the 4-dimethylaminocrotonic acid.
U.S. Patent No. 7,126,025 also discloses a process for the preparation of 4-dimethylaminocrotonic acid by treating methyl or ethyl 4-bromocrotonate with dimethylamine to provide methyl or ethyl 4-dimethylaminocrotonate, which is hydrolyzed to provide the 4-dimethylaminocrotonic acid.
U.S. Publication No. 2012/0046494 discloses a process for the preparation of 4-dimethylaminocrotonic acid or its salts by converting alkyl 4-chloro-3 -hydroxy butyrate to alkyl 4-hydroxy crotonate, which is brominated to obtain alkyl 4-bromo crotonate. The alkyl 4-bromo crotonate is treated with dimethyl amine to provide alkyl 4-dimethylaminocrotonate, which is hydrolyzed to get the 4-dimethylaminocrotonic acid.
The use of pyridine or carbon tetrachloride is toxic to humans and therefore their use for the manufacture of a drug substance is not advisable. The bromocrotonate compounds, being lachrymatory in nature, are difficult to handle on an industrial scale.
The present invention provides a faster, more efficient, and industrially feasible process for the preparation of 4-dimethylaminocrotonic acid of Formula II, which is used as an intermediate for the preparation of afatinib or its salts.
A first aspect of the present invention provides a process for the preparation of 4-dimethylaminocrotonic acid of Formula II or its salts,

Formula II
comprising the steps of:
i) converting 2,2-diethoxy-N,N-dimethylethanamine of Formula III

Formula III
to ethyl-4-(dimethylamino)crotonate of Formula IV; and

Formula IV
ii) hydrolyzing the ethyl-4-(dimethylamino)crotonate of Formula IV.
A second aspect of the present invention provides a process for the preparation of afatinib of Formula la or its salts,

Formula la
comprising the steps of:
i) converting 2,2-diethoxy-N,N-dimethylethanamine of Formula III

Formula III
to ethyl-4-(dimethylamino)crotonate of Formula IV;

Formula IV
ii) hydrolyzing the ethyl -4-(dimethylamino)crotonate of Formula IV to obtain 4- dimethylaminocrotonic acid of Formula II or its salts; and

Formula II
iii) converting the 4-dimethylaminocrotonic acid of Formula II or its salts to afatinib of Formula la or its salts.
EXAMPLES
Example 1 : Preparation of ethyl-4-(dimethylamino)crotonate (Formula IV)
In a round bottom flask, 2,2-diethoxy-N,N-dimethylethanamine (Formula III, 200 g) and deionized water (100 mL) were added at about 20°C to about 25°C. To the solution, concentrated hydrochloric acid (240 mL) was added at about 25°C to about 50°C. The temperature of the reaction mixture was raised to about 70°C. The reaction mixture was stirred at about 60°C to about 70°C for about 12 hours. The reaction mixture was cooled to about 0°C. To the reaction mixture, about 200 mL of aqueous potassium hydroxide (240 g in 250 mL water) was added at about 0°C to about 10°C to attain a pH of 9.0. To the reaction mixture, ethyl(diethoxyphosphoryl) acetate (200 g) and 2-methyltetrahydrofuran (600 mL) were added at about 0°C to about 5°C. Further, 50 mL of aqueous potassium hydroxide was added to the reaction mixture at about -5°C to about 0°C to attain a pH of about 13.5. The reaction mixture was stirred at about -5°C to about 0°C for about 1 hour. The reaction mixture was filtered, and then the filtrate was recovered under vacuum at about 45°C to about 50°C to obtain ethyl-4-(dimethylamino)crotonate as an oily mass.
Yield: 89%
Example 2: Preparation of 4-dimethylaminocrotonic acid hydrochloride (Formula ID
In a round bottom flask, ethyl -4-(dimethylamino)crotonate (Formula IV, 120 g) and ethanol (480 mL) were added at about 25°C to about 35°C. To the solution, aqueous sodium hydroxide (30.5 g in 60 mL water) was added at about 10°C to about 20°C. The temperature of the reaction mixture was raised to about 50°C. The reaction mixture was stirred at about 50°C to about 55°C for about 1 hour. The reaction mixture was cooled to about 5°C. To the reaction mixture, concentrated hydrochloric acid (120 mL) was added to attain a pH of 1.5. The reaction mixture was filtered on Celite® and washed with ethanol (50 mL). The filtrate was recovered under vacuum at about 55°C to about 60°C to obtain a crude mass. Ethanol (240 mL) was added to the crude mass, and then the reaction mixture was stirred at about 55°C to about 60°C for about 15 minutes to obtain a solution. In the solution, sodium chloride was obtained as a byproduct. The solution was filtered to discard sodium chloride. The filtrate was recovered under vacuum at about 55°C to about 60°C to obtain a residue. To the residue, isopropanol (400 mL) was added, and then the reaction mixture was stirred at about 55°C to about 60°C to obtain a clear solution. The solution was gradually cooled to about 25°C to about 30°C. The solution was further stirred at the same temperature for about 2 hours. The solid obtained was filtered, and then washed with isopropanol (50 mL). The solid was dried under vacuum at about 55°C to about 60°C to provide 4-dimethylaminocrotonic acid hydrochloride.
Yield: 63%
Sun Pharma managing director Dilip Shanghvi.
//////////

No comments:
Post a Comment